Ferroptosis is a regulated cell death process triggered by the toxic accumulation of lipid peroxides on cell membranes. This iron-dependent form of cell death distinguishes itself from other mechanisms, like apoptosis. Lipid peroxides, products of routine metabolic processes, induce oxidative damage to cell membranes, contributing to the distinctive features of ferroptosis.
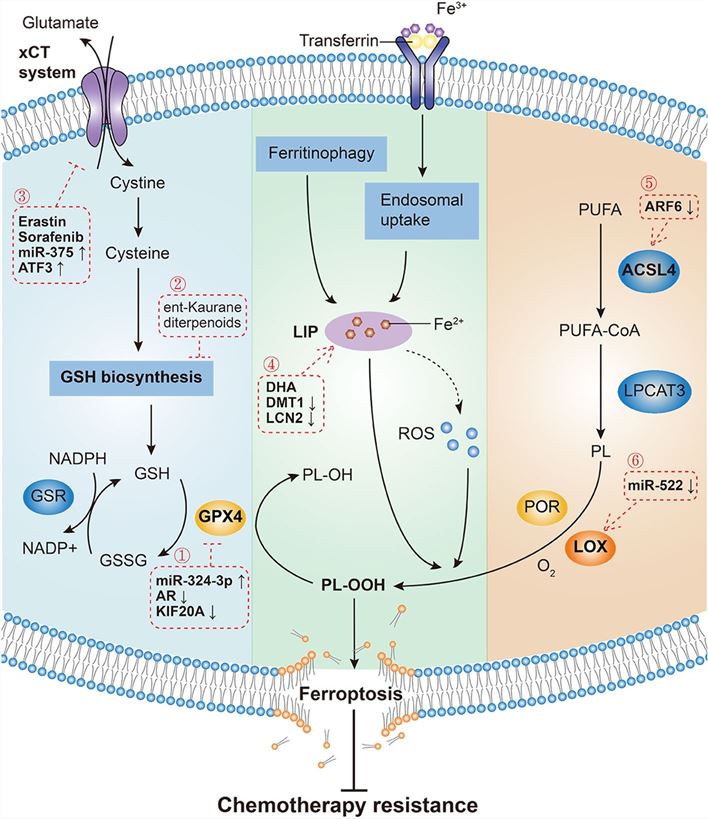 Figure 1. Mechanisms governing ferroptosis and reversing chemotherapy resistance. Three pathways initiate the process of ferroptosis and chemotherapy resistance reversal: the canonical GPX4-regulated pathway, iron metabolism pathway and lipid metabolism pathway.[1]
Figure 1. Mechanisms governing ferroptosis and reversing chemotherapy resistance. Three pathways initiate the process of ferroptosis and chemotherapy resistance reversal: the canonical GPX4-regulated pathway, iron metabolism pathway and lipid metabolism pathway.[1]
One extensively researched aspect of ferroptosis is its involvement in cancer. Ferroptosis exhibits dual effects, acting both pro-tumor and anti-tumor. Ferroptosis can promote tumor growth by causing the death of immune cells that would normally hinder cancer progression. Additionally, the heightened production of reactive oxygen species (ROS) during ferroptosis can also stimulate tumor cell proliferation. However, cancer cells are vulnerable to ferroptosis due to their elevated levels of iron and ROS, resulting from their increased metabolic activity for uncontrolled cell growth. Numerous studies have demonstrated that inducing ferroptosis in cancer cells can be a successful method for selectively eliminating them, while minimizing harm to surrounding healthy cells.
Creative Bioarray offers a comprehensive ferroptosis assay, a cutting-edge solution for exploring and understanding the intricate mechanisms of cell death. Our assay provides a sophisticated platform to investigate ferroptosis induction and its impact on various cell types.
References:
1. Zhang, Chen et al. "Ferroptosis in cancer therapy: a novel approach to reversing drug resistance." Molecular cancer vol. 21,1 47. 12 Feb. 2022, doi:10.1186/s12943-022-01530-y
2. Li, Jie et al. "Ferroptosis: past, present and future." Cell death & disease vol. 11,2 88. 3 Feb. 2020, doi:10.1038/s41419-020-2298-2
3. Murray MB, Leak LB, Lee WC, Dixon SJ. Protocol for detection of ferroptosis in cultured cells [published online ahead of print, 2023 Aug 8]. STAR Protoc. 2023;4(3):102457. doi:10.1016/j.xpro.2023.102457
Online Inquiry