Cardiac fibrosis, a prevalent characteristic in several cardiovascular diseases, is marked by exaggerated production and deposition of extracellular matrix (ECM) components, particularly collagens, in the heart's connective tissue. This results in increased stiffness and decreased contractility, ultimately leading to a decline in cardiac function.
Human cardiac fibroblasts are the primary cells responsible for maintaining the structure and function of the heart. They synthesize and secrete extracellular matrix proteins, such as collagen and fibronectin, which provide mechanical support to the heart muscle. Additionally, cardiac fibroblasts play a critical role in the repair process following myocardial injury.
Transforming Growth Factor-beta (TGF-β) is a key signaling molecule implicated in the induction of fibrosis, including cardiac fibrosis. When subjected to TGF-β treatment, human cardiac fibroblasts undergo several changes in their gene expression and phenotype. TGF-β signaling pathway activation leads to the stimulation of fibroblast proliferation, migration, and the synthesis of extracellular matrix proteins. This process is crucial for tissue repair and scar formation in response to cardiac injury or remodeling.
At Creative Bioarray, we are committed to providing researchers with reliable and high-quality cell-based tools for advancing drug discovery efforts. Our TGF-β-induced human cardiac fibroblasts can help researchers better understand the mechanisms of cardiac fibrosis and discover potential therapeutic options for the treatment of related cardiovascular diseases.
 Figure 1. TGF-β pathway screening and profiling
Figure 1. TGF-β pathway screening and profiling
Study Examples:
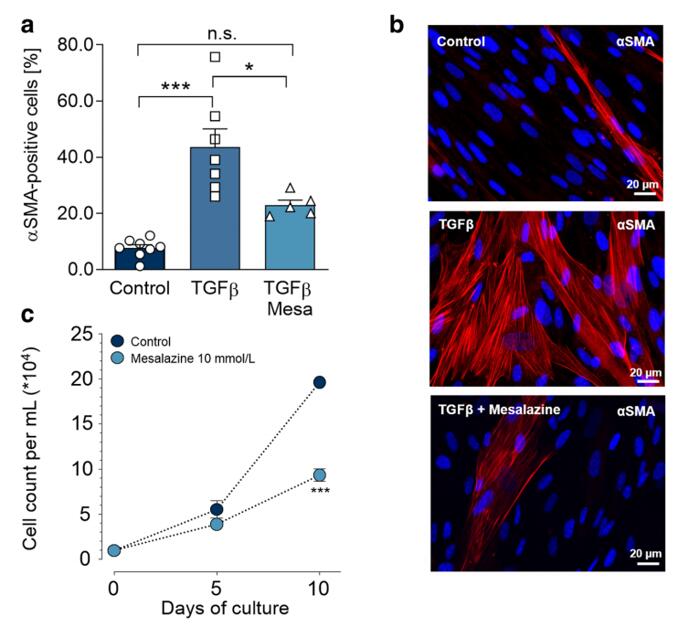 Figure 2. Mesalazine reduces TGFβ-induced myofibroblast differentiation and fibroblast proliferation. [1]
Figure 2. Mesalazine reduces TGFβ-induced myofibroblast differentiation and fibroblast proliferation. [1]
Reference:
1. Hoffmann, Maximilian et al. "Repurposing mesalazine against cardiac fibrosis in vitro." Naunyn-Schmiedeberg's archives of pharmacology vol. 394,3 (2021): 533-543. doi:10.1007/s00210-020-01998-9
Online Inquiry