Human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes (CMs) are a valuable tool for drug discovery and cardiac disease modeling. One of the key functional assays used to assess the maturation and functionality of hiPSC-CMs is the measurement of calcium transients.
Calcium serves as a key signaling molecule that mediates the communication between the action potential and cardiomyocyte contraction. When there are elevated levels of calcium within the cell, the cardiomyocyte contracts, while lower levels of calcium lead to relaxation. Alterations in the activity of ion channels responsible for generating the action potential can result in changes to the shape and duration of the intracellular calcium transient. This refers to the fluctuation in calcium concentrations within the cell during each cardiac cycle. Consequently, by examining the impact of different compounds on the intracellular calcium transient, researchers can evaluate their effects on cardiac function.
To perform calcium transient assays, hiPSC-CMs are typically plated on a glass coverslip or a multi-well plate and loaded with the calcium indicator dye. The cells are then stimulated to contract and the fluorescence signals are detected using fluorescence microscopy or plate readers equipped with fluorescence detection capabilities.
Creative Bioarray has developed and validated a high-throughput assay in hiPSC-CMs using a Ca2+ sensitive dye and the FLIPR Screening System to monitor intracellular Ca2+ oscillation in response to test compounds.
Study Examples:
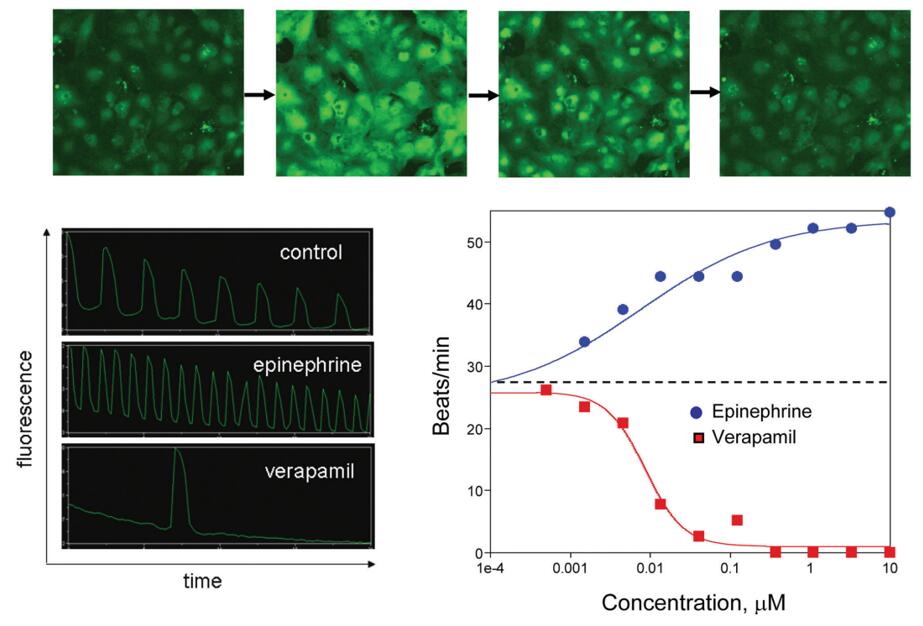 Figure 1. Top: Images of cardiomyocytes showing low and high Calcium 5 signal through beat cycles. Lower Left: Visualizing beating of cardiomyocytes by fluorescence intensity (Calcium 5 signal). Lower Right: Temporal response of signals from cells dosed with positive and negative chronotropes. [1]
Figure 1. Top: Images of cardiomyocytes showing low and high Calcium 5 signal through beat cycles. Lower Left: Visualizing beating of cardiomyocytes by fluorescence intensity (Calcium 5 signal). Lower Right: Temporal response of signals from cells dosed with positive and negative chronotropes. [1]
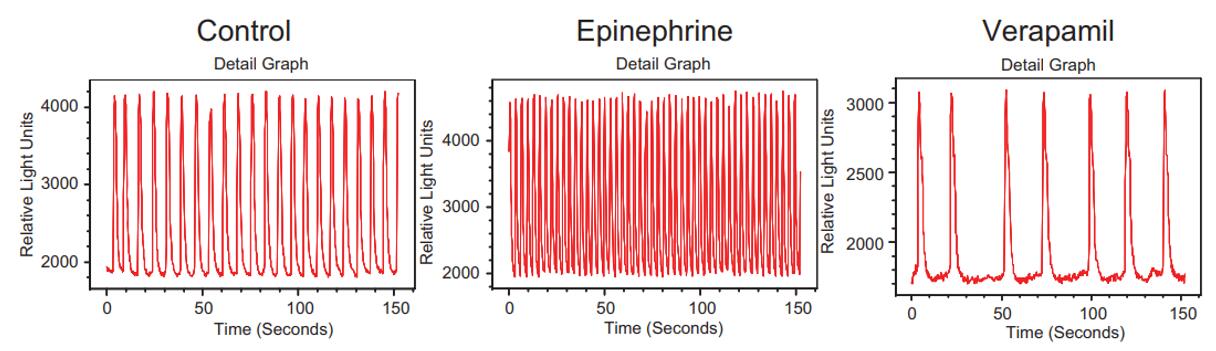 Figure 2. Fluorescence intensity versus time for three representative wells of beating cardiomyocytes loaded with FLIPR Calcium 5 dye. Measurement was done on a FLIPR Tetra system. [1]
Figure 2. Fluorescence intensity versus time for three representative wells of beating cardiomyocytes loaded with FLIPR Calcium 5 dye. Measurement was done on a FLIPR Tetra system. [1]
Reference:
1. Sirenko, Oksana et al. "Multiparameter in vitro assessment of compound effects on cardiomyocyte physiology using iPSC cells." Journal of biomolecular screening vol. 18,1 (2013): 39-53. doi:10.1177/1087057112457590
Online Inquiry